Developing High-Quality FDA-Compliant Retort Processes with Scientific Validation
This ultimatte guide breaks down how to build FDA-compliant retort systems, develop scientifically validated processes, and fine-tune recipes so your shelf-stable foods taste as fresh as the day they were made.
For manufacturers of ready-to-eat (RTE) products, ensuring that every pouch, can, or tray leaves the factory both microbiologically safe and appealing to eat is the ultimate goal. Achieving this balance requires more than a well-built retort; it demands a scientifically validated process, tailored to the unique behavior of the equipment, the packaging, and the product itself.
This guide explains how FDA-compliant retort systems are structured, why validation is critical, and how proper process design can help manufacturers consistently deliver products that meet global regulatory standards while maintaining outstanding quality.
Part 1: Setting Up an FDA-Compliant Retort System
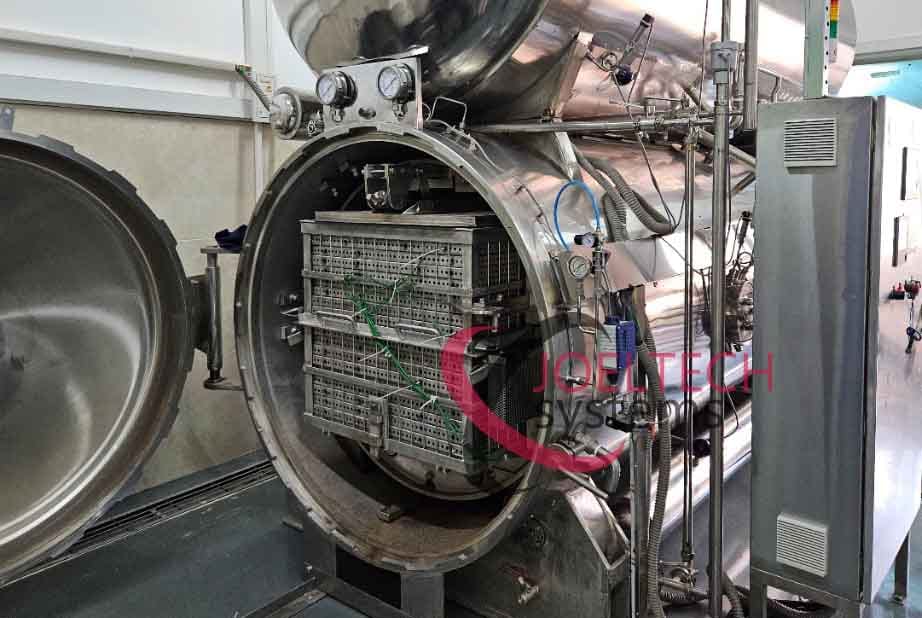
A retort is not simply a large pressure cooker, it’s an automated processing system that must demonstrate repeatability, accuracy, and safety under all conditions. An FDA-compliant retort must control, record, and document every cycle with minimal human intervention. Let’s examine the essentials.
Automatic Control Through PLCs
All critical parameters: temperature (T), pressure (P), water level, and flow should be governed by a programmable logic controller (PLC). Once a cycle begins, operators should not intervene manually. An automated system ensures every cycle follows the same programmed path, delivering consistent results batch after batch.
Comprehensive Recording and Documentation
Every retort cycle generates critical data. FDA requires that temperature, pressure, and flow records be retained for verification. This data becomes the official record of safety. Manufacturers carry the responsibility of maintaining complete, accurate batch records
High Quality Control and Reference Sensors
The sensors used in retort systems are the heart of the process. Both control sensors (which regulate the process) and reference sensors (which confirm accuracy) must be of the highest quality. A low-grade sensor can easily misreport conditions, leading to under-processed food or unnecessary over-processing.
PID Control and Temperature Stability
A proportional–integral–derivative (PID) controller provides precise regulation of steam or water flow to the chamber, smoothing out temperature fluctuations. FDA expects uniformity across the chamber ,no more than ±1 °C deviation during the holding phase. Achieving this consistency ensures that every container, whether at the center or edge of the load, experiences the required lethality.
Flow and Water Level Monitoring
Additional instruments, such as flow meters and water level indicators, enhance reliability. They ensure the chamber environment is predictable, repeatable, and safe.
While many retorts international or domestic, can meet the FDA’s baseline requirements, not all perform equally. Differences in component quality, system design, and control sophistication influence how well the equipment maintains uniform conditions. For manufacturers, this means the equipment is only part of the equation. The way processes are developed, validated, and controlled is just as critical.
A perfectly optimized process, tailored to the retort’s behavior, can yield products nearly indistinguishable from freshly prepared equivalents. But this outcome requires more than a good quality retort; it requires careful validation and data-driven scientific process design.
Part 2: Developing FDA-Compliant Retort Processes
Even with a compliant retort, product quality and safety depend on how the process is established. Every retort, every product, and every container configuration is unique. There is no universal recipe you can borrow from another plant because it works for them. Attempting to apply a “standard” process across equipment and products is a recipe for compromise. Scientific validation ensures that safety is guaranteed without sacrificing quality.
Temperature Distribution Studies
The first step is understanding how heat moves inside the retort chamber. A temperature distribution study (TD study) maps cold spots (the least heated areas) and hot spots (the most heated areas). These points serve as references for validation and quality checks:
- Cold spots determine the slowest-heating location, critical for ensuring sterilization. Samples for microbiological testing should always be drawn from here.
- Hot spots receive the most heat and are most prone to overcooking. Samples taken here provide insights into product quality, taste, and texture variations across a batch.
By comparing results from both zones, manufacturers gain a clear picture of product consistency.
Defining Initial Temperature
The initial product temperature (IT) before processing is fundamental. A hot-filled product enters at 80–90 °C and needs much less come-up time than a room-temperature fill at 35 °C. A cold fill, meanwhile, demands a longer heating curve.
So, the processor should keep this in mind while developing the retort program. Without accurate definition of IT, processes risk under-sterilization or over-processing. Once defined through studies, manufacturers must clearly define the initial temperature and enforce it rigorously

Determining Operating Temperature
The retort’s operating temperature (set point) must be chosen based on distribution data. In practice, this often means setting the operating temperature slightly higher than the target process temperature, typically by 0.5–1 °C, to ensure all areas of the retort meet or exceed the required threshold. Factors such as container type, load configuration, and retort quality all affect this offset.
The Cooling Cycle
Cooling may seem secondary, but it plays a critical role in product safety and packaging integrity. During heating, steam/gases from the product build up inside containers. If cooling is too rapid or uneven, containers can deform or even rupture. Common issues include:
- Can buckling or paneling in metal cans
- Bulging or wrinkling in pouches
- Seal failures in semi-rigid containers
A validated cooling profile accounts for container type, product characteristics, and cooling water temperature. Only through validation can manufacturers prevent these costly defects.
Loading and Capacity
Loading patterns significantly impact heat transfer. Overloading, or assuming one process works across all container types, is a serious mistake. Validation must be conducted for each configuration; different container sizes, shapes, and weights each alter heat distribution and has different heating capacities. For safe sterlization in every batch, the process for each product type should only be set based on validation data.
Partial load studies can also be very useful, necessary when manufacturers plan to process reduced batches.
Part 3: Heat Penetration Studies: The Core of Process Validation
This is the most critical step in process validation. Temperature distribution defines how the chamber behaves; heat penetration studies (HP studies) reveal how the product itself heats.
HP studies examine how quickly heat penetrates to the coldest point within a container. The retort process must be designed so that this point achieves the required sterilization value (Fo). Safety always takes precedence, but quality is protected by avoiding unnecessary over-processing. The critical factors of a particular product, liike pH, water activity, ingredient types etc must be studied carefully and only based on this data, will we establish the retort process.
Establishing a Safe Thermal Process
The first priority of every HP study is to confirm that the process delivers sufficient Fo value (a measure of sterilization) at all points in the product, particularly at the cold spot. Achieving this guarantees product safety against spore-forming pathogens such as Clostridium botulinum.
To ensure robustness, worst-case scenarios are examine hered. Test packs are loaded at the coldest retort locations, with sensors positioned at the container’s cold spot. Products are prepared at the minimum expected IT to challenge the process. By validating under these harshest conditions, manufacturers guarantee safety under all real-world variations.
Accuracy of Equipment
HP studies are highly sensitive. A one-degree Celsius error at 121 °C can cause a 26% deviation in calculated Fo values. Such an error can result in unsafe processes or unnecessary degradation of product quality. This underlines why high-precision validation tools are indispensable.
For this reason, it is strongly recommended that these studies be carried out only with high-precision validation solutions such as the Tracksense Pro systems from Ellab A/S, Denmark. These systems provide unmatched accuracy, reliability, and traceable documentation required for FDA compliance.
Proper Validation Accessories
Proper accessories are often an underlooked part of the validation system. But these are critical. If validation fittings are not correctly designed, there is a high risk of steam or water ingression into the product, which alters the heating curve and results in inaccurate Fo values. Such errors can invalidate the entire study.
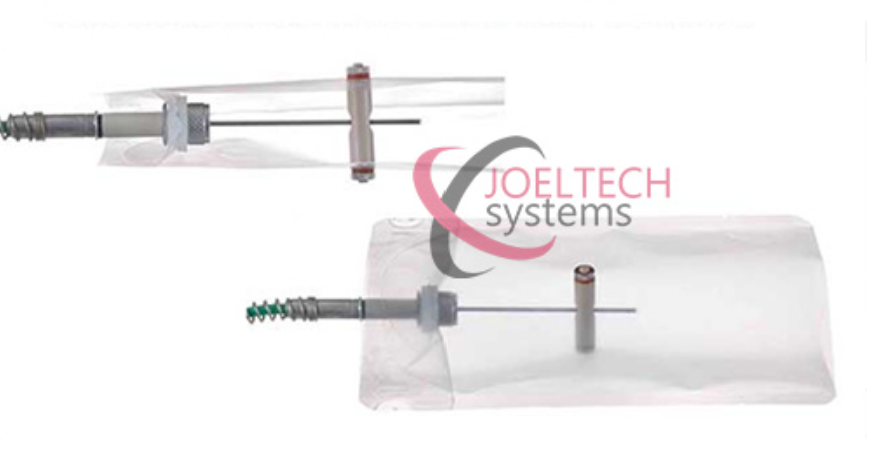
Ellab is currently the only provider offering purpose-built validation fittings designed for all container types and sizes. These accessories ensure the integrity of data and make HP studies reliable.
Role of the Process Authority
As per FDA guidelines, all Heat Penetration and process standardization studies should be conducted under the supervision of a certified Process Authority. Independent, qualified experts ensure that the designed process is not only scientifically robust but also meets regulatory requirements. FDA and international authorities rely on this independent certification as proof of compliance.
How We Help
Joeltech Systems provides world-class process standardization and validation services, helping manufacturers produce safe, high-quality retorted foods with complete regulatory compliance. All validation studies are conducted using sophisticated Ellab A/S solutions from Denmark, ensuring accurate, reproducible, and globally recognized data.
Beyond data collection, Joeltech Systems works collaboratively with clients to design retort processes that balance 100% safety with the highest possible product quality. Using advanced solutions from Ellab A/S Denmark, we ensure accurate studies, reliable data, and scientifically designed processes that meet the highest global standards. This means not just meeting FDA requirements, but delivering foods that maintain their sensory appeal and market competitiveness.