Conducting the Perfect Retort Validation.
Essential Role of Ellab Equipment in Retort Validation Services
 Even the smallest systematic or experimental error during the validation studies can result in significant financial losses, potentialy millions of dollars to the processors as the regular commercial production fully depends on the control parameters derived from the validation data. Improper validation techniques, use of unreliable equipment, use of improper heat penetration fittings, usage of unapproved validation software etc are some of the reasons for the wrong validation process
Even the smallest systematic or experimental error during the validation studies can result in significant financial losses, potentialy millions of dollars to the processors as the regular commercial production fully depends on the control parameters derived from the validation data. Improper validation techniques, use of unreliable equipment, use of improper heat penetration fittings, usage of unapproved validation software etc are some of the reasons for the wrong validation processCritical Factors That Determine the Efficacy of the Process
The most critical considerations in the development of processes for heat preserved foods is the acquisition of accurate time / temperature profiles. The main reasons for this are :1. Safety
Ensuring that microbial safety has been achieved and there is no risk of food poisoning or food spoilage
2. Optimization
Reducing process times to increase production throughput and minimize energy costs.
3. Improvement of Quality
Optimization of vitamin and protein retention, and product sensorial qualities.
Process Validation
Providing documentation to demonstrate compliance with regulations for authorities , FDA / USDA, EC, customers, ISO, HACCP etc. The validation procedure encompasses many aspects with the process vessel and product both needing detailed study. In both cases, it is important to find the cold zone at which the slowest heat transfer will be applied. Three phases of tests should be considered :Temperature Distribution : Temperature mapping within a fully loaded process vessel to investigate performance against a control program and identify the cold zone.
Cold Point Determination :Multiple measurements within a product container to find the slowest heating point within the product. This will be product and packaging dependent.
Heat Penetration : Replicate measurements with temperature measurement devices located at the position identified within the cold point tests.
A systematic or experimental error of 1 deg C in a temperature measurement system at the sterilization reference temperature of 121.1 deg C, would lead to a corresponding error of 26% in the calculated Fo sterility value. It is therefore important that the correct equipment with proper fittings is applied for a given validation. Ellab offer a collection of large selection probes, sensors, packing glands and tools which are available for correct mounting in many styles and designs of containers.
Heat Penetration Accessories from Ellab A/S Denmark

 The range of heat penetration fittings are selected based on the following parameters taken into consideration :
The range of heat penetration fittings are selected based on the following parameters taken into consideration :Correct Positioning of the Measuring Point :
It is very important that, the packing gland and probe are correctly positioned in the “cold spot”. If this is not obtained, it can result in “false” measurements as the temperature is collected not from the coldest zone in the product, risking high Fo / Po values.
Elimination of Steam and Water Ingression :
It is very important that the packing gland and the probe are mounted properly, so as to maintain the integrity of the container and ensure that water / steam ingression to the measuring point cannot occur leading to false data and high Fo/Po values.
The above photos show how Ellab sensors are fixed properly – air tight and water tight – to the containers, to eliminate steam and water ingression during measurement. In case, steam or water ingression occurs, false result will be shown with higher Fo values, resulting complete failure of the validation result.
Minimizing risks of Heat Conduction :
It is recommended that the probe and gland are mounted from the side of the container with the longest distance to the “ cold spot” , so that heat conduction from the water / steam is avoided.
Minimizing the Probe Assembly Inside the Container :
It is very important that the probe and gland are as small as possible so that the impact on the internal environment (product) of the container, including the head space is minimized. This is especially important for products that receive a rotary process where the movement of the headspace will be critical factor in the rate of heat transfer.


If it is a mixed product in liquid, it is important that the biggest piece of the solid is positioned at the measuring point, and that the probe assembly is mounted so it is not preventing the other solid pieces to move freely. The reference container must be as close to the “normal” containers as possible, otherwise the heat transfer profile will no longer ne representative for the entire batch.
Heat Penetration (H.P.) Study. How it is done ?
The photo shows how the core of the biggest piece of the product ( tender jack fruit pieces) inside the can is firmly positioned at the measuring sensor tip to measure the core temperature of the hardest piece, placed at the cold spot of the can, which is placed at the cold spot of the retort The packing gland is fixed air tight and water tight with proper fittings and washers, eliminating steam and water ingression. The sensor needle is inserted through the packing gland after filling and seaming of the can to measure the core temperature of the product.
 This method ensures the following: -
This method ensures the following: -





The required holding / cooking / sterilization time for that recipe can be established in the retort PLC , based on the time taken to attain the required Fo value to the above mentioned sample can, in conjunction with corresponding TD profile.
Why Accurate and Reliable Sensors with proper Fittings are mandatory for HP Studies ?
In case of an under-sterilization issue occurred in the plant, the processor will realize the batch failure only after getting the red signals from the microbiology lab / incubation centre. The time duration for getting this result is about 10 days to 2 weeks from the production date / incubation date.
Assume that the daily production of a retort company is 100,000 pouches. By the time, the processor gets the rejection warning from the testing lab for the batch which completed the incubation period, he might have completed the production of almost 1.4 million pouches. The entire production of 1.4 million pouches needed to be discarded in case of a rejection warning of the products of the first batch. The loss will be millions of Dollars due to the false validation study and implementation of the wrong data.
It is observed that, some processors confidently export the products even before waiting for the incubation period. Such processors will be in big trouble, in case of such batch failure.
A systematic or experimental error of 1 deg C in a temperature measurement system at the sterilization reference temperature of 121.1 deg C, would lead to a corresponding error of 26% in the calculated Fo sterility value.
Eg. For processing at 121.1 deg C, with z value as 10 deg C, the Fo value obtained by the product with internal temperature 121 deg C for 1 minute is 0.974. Fo value achieved at 122 deg C for 1 minute is 1.227. Assume that the core of product attained temperature of 121 deg C for 10 minutes during holding / sterilization time. Then the Fo value obtained by the product will be 0.974 x 10 = 9.74.

If due to the wrong measurement of the heat penetration data ( due to any of the earlier mentioned reasons) , if the sensor for the sample products showed 122 deg C ( instead of actual 121 deg C) , then the Fo value achieved will be 10 x 1.227 = 12.27 which is almost 26.7% higher than the actual value. Here we can observe that, the difference in Fo value even for 10 minutes holding is 2.27. Customers normally process the products with 30 to 35 minutes holding. In such case, the Fo value difference will be very high and the processing / products will be in danger.
So it is mandatory to perform the Retort Validation through companies / persons who are expert in process establishment and also uses high accurate and reliable sensors with correct fittings and fixtures, suitable to the container geometry for Heat Penetration Studies.
 The photo shows Ellab wireless Datalogger with Heat Penetration Fittings for Pouches and Cans
The photo shows Ellab wireless Datalogger with Heat Penetration Fittings for Pouches and CansWhy FDA insist to use Validation software which is in compliance with FDA 21 CFR part 11 ?
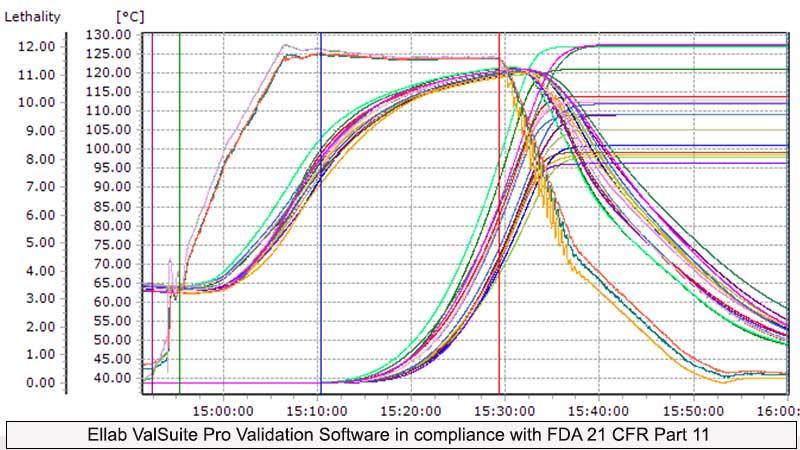
Ellab ValSuite Pro Software is validated software, fully in compliance with FDA 21 CFR Part 11. No modification or manipulation is allowed in the collected data, ensuring trustworthy and reliable data. Complete details including the serial numbers of the sensors, measurement and operator details etc are documented with proper authentication and electronic signature.